The availability of MRI in clinical and laboratory settings at our institute offers an ideal means for translational research in patients and clinically relevant animal models of brain disorders. Multiparametric information on vessel patency (MR angiography), blood flow (perfusion MRI) and tissue damage (structural MRI) can be obtained in a single session. We are applying various MRI methods to get improved insights in brain pathophysiology, and to test novel treatment strategies in different rodent models of brain disorders, such as stroke and epilepsy. Furthermore, we are developing machine learning strategies for tissue classification and outcome prediction based on multiparametric MRI data. These tools, which are also being tested in clinical settings, may help to improve diagnosis and treatment decision making.
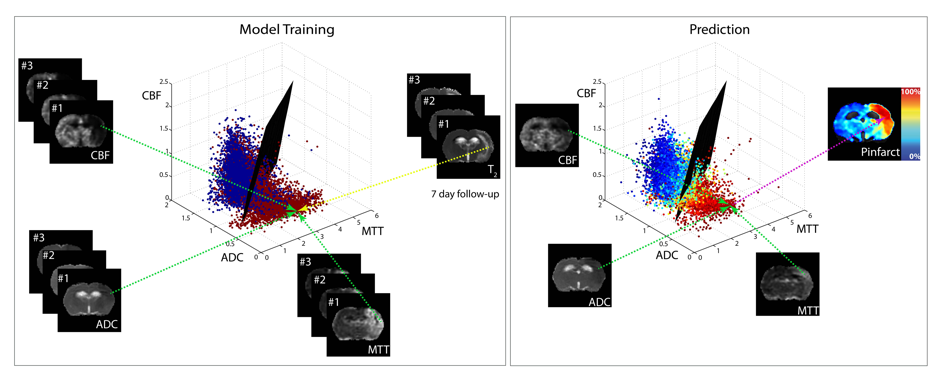
Multiparametric MRI data of brain tissue status (from maps of cerebral blood flow (CBF), apparent diffusion coefficient (ADC) and mean transit time (MTT)) in different rats with a unilateral ischemic stroke. For model training for outcome prediction, acute MRI data were voxel-wise related to tissue outcome (i.e. infarcted (red) or non-infarcted tissue (blue)) based on follow-up T2 scans or histology. Subsequently a generalized linear model (GLM)-based statistical algorithm estimated a separating plane to differentiate between infarcted and non-infarcted voxels. For tissue outcome prediction in another stroke subject, the distribution pattern of the newly introduced voxel-wise combinations relative to the separating plane can be used to estimate infarction risk (0% ≤ Pinfarct ≤ 100%). Courtesy of Mark Bouts.
Cellular/molecular imaging
Recent advances in contrast agent design – allowing agents to be long-circulating, cell-loadable and/or targeted – have enabled measurement of tissue vascularity, cellular infiltration and endothelial markers with MRI. This requires interdisciplinary cross-talk, for which we have teamed up with (inter)national experts in nanotechnology, cellular and molecular biology, and pharmacology.
Cell labeling with superparamagnetic contrast agents – under in vivo or in vitro conditions – allows MRI-based detection of labeled cells. This approach can be employed to measure infiltration of leukocytes, like monocytes and lymphocytes, which play a critical role in brain pathophysiology, but it may also be of use to monitor delivery and fate of stem cells in cell-based therapies.
MRI of molecular entities is feasible with targeted contrast agents. Targeting can be accomplished by conjugation of a specific ligand – e.g. an antibody – to a contrast agent complex. We have shown that endothelial markers, such as cell adhesion molecules involved in binding and infiltration of circulating leukocytes, can be detected following intravascular injection of selectively binding contrast agents.
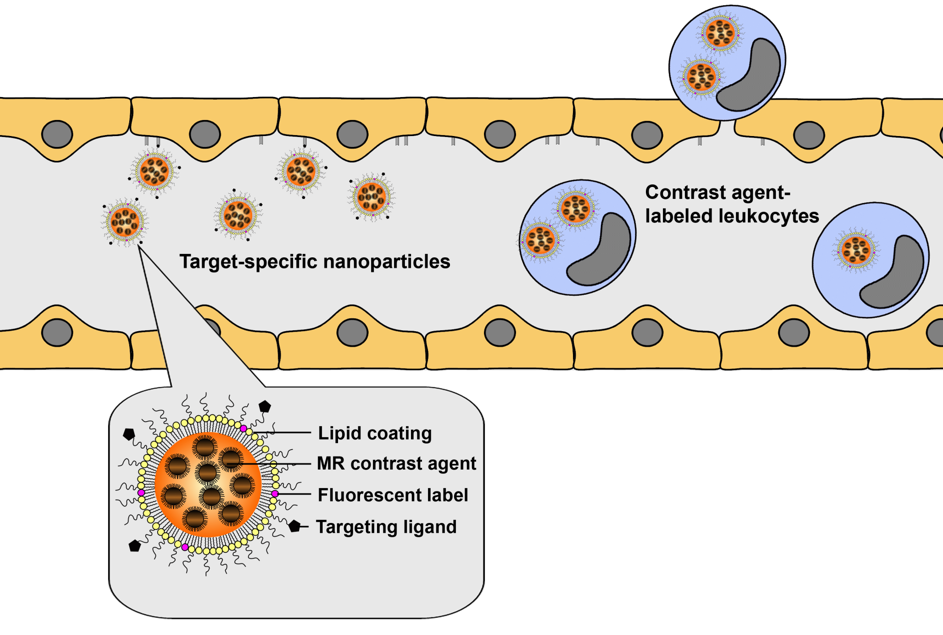
Schematic overview of the concept of cellular and molecular MRI of neuroinflammation. Target-specific contrast agents allow detection of upregulated endothelial markers, such as cell adhesion molecules, while infiltrating leukocytes can be detected after specific cell labeling with contrast agents. Multimodal lipid-based nanoparticles with incorporated MR contrast agent and fluorescent label can be designed for combined MRI and optical imaging of cells or molecular markers. Courtesy of Geralda van Tilborg.
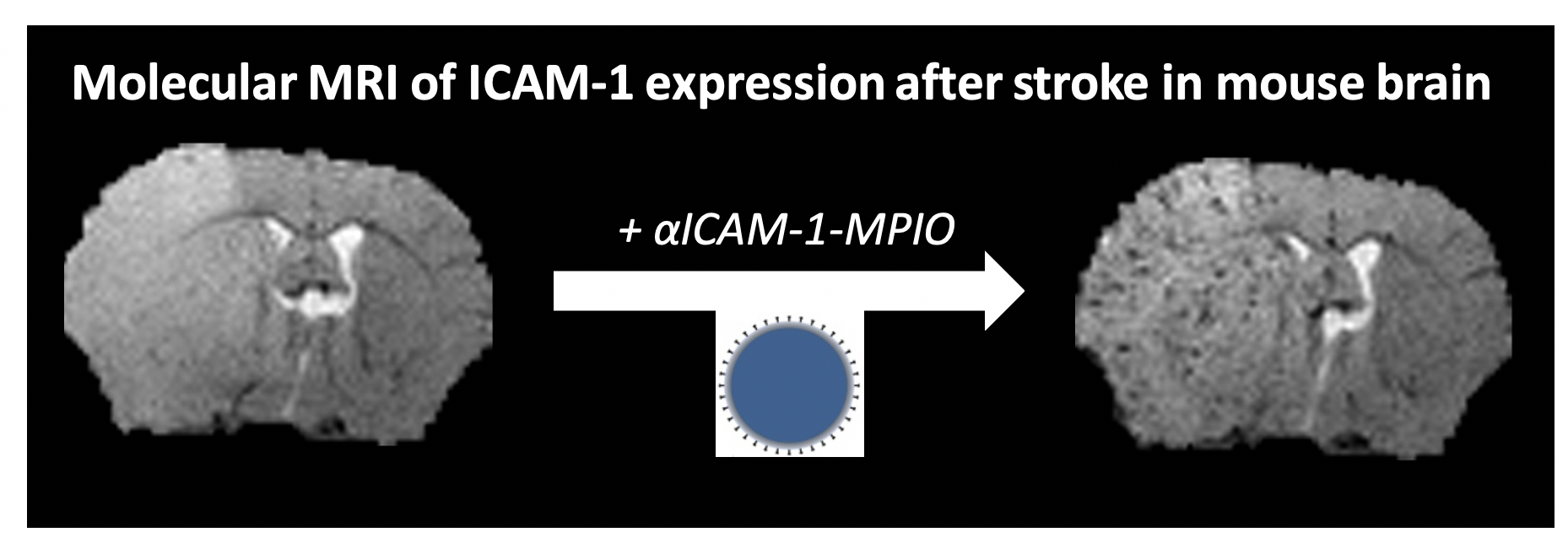 T2*-weighted MR images of a mouse brain slice before and 1 h after injection of micron-sized particles of iron oxide coated with antibodies against intercellular adhesion molecule-1 (αICAM-1-MPIO), at 24 h after ischemic stroke. The lesion is characterized by edema-associated signal increase in pre- and post-contrast images, while a vascular pattern of hypointensities, reflecting neurovascular inflammation, appeared after αICAM-1-MPIO injection. Courtesy of Lisette Deddens.
T2*-weighted MR images of a mouse brain slice before and 1 h after injection of micron-sized particles of iron oxide coated with antibodies against intercellular adhesion molecule-1 (αICAM-1-MPIO), at 24 h after ischemic stroke. The lesion is characterized by edema-associated signal increase in pre- and post-contrast images, while a vascular pattern of hypointensities, reflecting neurovascular inflammation, appeared after αICAM-1-MPIO injection. Courtesy of Lisette Deddens.
Contrast-enhanced MRI methods provide powerful means to assess (changes in) hemodynamic indices, vascular parameters and inflammatory markers, with the potential of identifying critical biomarkers and therapeutic targets. These approaches thereby broaden the already impressive arsenal of MRI methods for experimental and (pre)clinical assessment of brain diseases and therapies.
MR microscopy
MRI of ex vivo or post mortem tissue samples at high magnetic field strength (≥7 T) and with long acquisition time provides unique opportunities for detailed (microscopic) histological and morphometric assessment, as well as sensitive detection of (individual) labeled cells or molecular markers. For example, we have obtained anatomical images of rat brain and human vessel structures at spatial resolutions of <50 μm, offering great histological/histopathological detail.
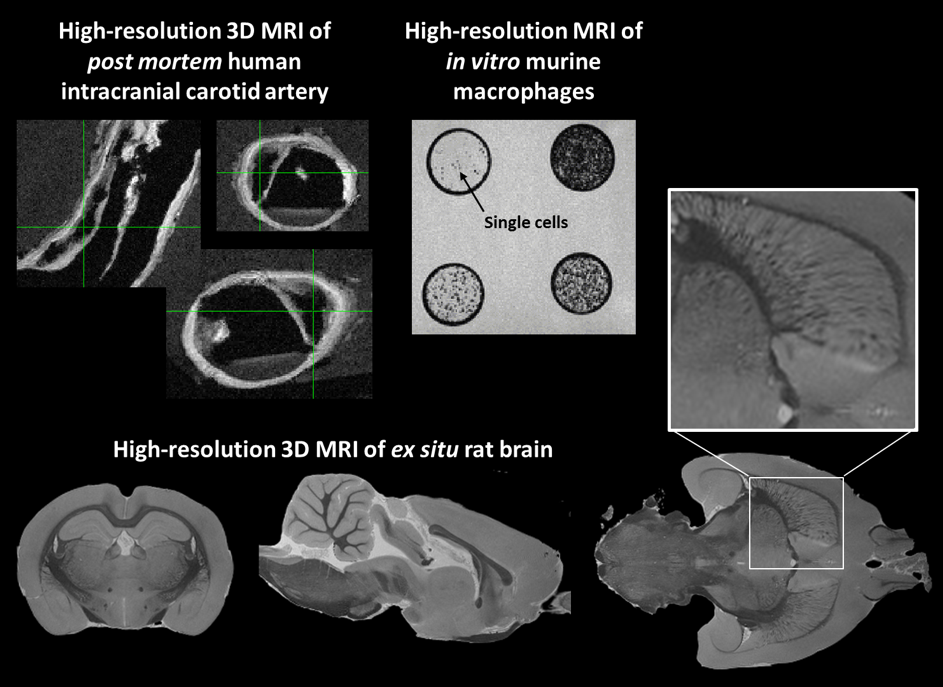
Ultrahigh-resolution post mortem/in vitro/ex vivo MRI of a human patient’s intracranial carotid artery (balanced steady-state free procession (bSSFP) scan; isotropic 75 μm voxel size), murine macrophages labeled with iron oxide nanocrystals (gradient echo MRI; isotropic 100 μm voxel size), and rat brain (gradient echo scan; isotropic 67 μm voxel size), obtained with our preclinical 9.4 T MR system. Individual murine cells (arrow), histopathological details of an atherosclerotic plaque with intimal thickening and calcification (green cross-hair), and white matter structures from the rat internal capsule through the basal ganglia (zoomed insert) are clearly evident. Courtesy of Remko Kockelkoren, Geralda van Tilborg and Annette van der Toorn.
Advantages of post mortem/ex vivo MRI as compared to conventional histology include absence of tissue processing artefacts, reduced time and labor for acquisition, unique and adjustable tissue contrasts, and straightforward digital 3D assessment. This can be exploited for digital brain atlasing and morphological phenotyping, enabling accurate anatomical comparison within and between experimental groups.

Post mortem MRI allows morphometric analysis of detailed changes between experimental groups, for instance to assess neurodevelopment. Top left: T2-weighted MR images, and fractional anisotropy and mean diffusivity maps (obtained from DTI) of post mortem rat brain. Registration of anatomical images to an average rat brain template allows deformation-based morphometric analysis (top right) or cortical thickness measurement (bottom right) to detect differences between experimental groups, in this case adult and adolescent brains. Differences between white matter structures can be analyzed by tract-based spatial statistics of DTI data (bottom left). Courtesy of Kajo van der Marel.
Relevant publications
In Vivo Molecular MRI of ICAM-1 Expression on Endothelium and Leukocytes from Subacute to Chronic Stages After Experimental Stroke. Deddens LH, van Tilborg GAF, van der Marel K, Hunt H, van der Toorn A, Viergever MA, de Vries HE, Dijkhuizen RM. Translational stroke research. 2017;8(5):440-448.
Valproate Reduces Delayed Brain Injury in a Rat Model of Subarachnoid Hemorrhage. Hamming AM, van der Toorn A, Rudrapatna US, Ma L, van Os HJ, Ferrari MD, van den Maagdenberg AM, van Zwet E, Poinsatte K, Stowe AM, Dijkhuizen RM, Wermer MJ. Stroke. 2017;48(2):452-458.
Longitudinal assessment of blood-brain barrier leakage during epileptogenesis in rats. A quantitative MRI study. van Vliet EA, Otte WM, Gorter JA, Dijkhuizen RM, Wadman WJ. Neurobiol Dis. 2014;63:74-84.
Imaging neuronal loss and recovery in compromised but viable brain tissue. Dijkhuizen RM. Brain. 2013;136(Pt 6):1689-91.
Early identification of potentially salvageable tissue with MRI-based predictive algorithms after experimental ischemic stroke. Bouts MJ, Tiebosch IA, van der Toorn A, Viergever MA, Wu O, Dijkhuizen RM. J Cereb Blood Flow Metab. 2013;33(7):1075-82.
MRI of ICAM-1 upregulation after stroke: the importance of choosing the appropriate target-specific particulate contrast agent. Deddens LH, van Tilborg GA, van der Toorn A, van der Marel K, Paulis LE, van Bloois L, Storm G, Strijkers GJ, Mulder WJ, de Vries HE, Dijkhuizen RM. Mol Imaging Biol. 2013;15(4):411-22.
Combined treatment with recombinant tissue plasminogen activator and dexamethasone phosphate-containing liposomes improves neurological outcome and restricts lesion progression after embolic stroke in rats. Tiebosch IA, Crielaard BJ, Bouts MJ, Zwartbol R, Salas-Perdomo A, Lammers T, Planas AM, Storm G, Dijkhuizen RM. J Neurochem. 2012;123 Suppl 2:65-74.
In vivo diffusion tensor imaging and ex vivo histologic characterization of white matter pathology in a post-status epilepticus model of temporal lobe epilepsy. van Eijsden P, Otte WM, van der Hel WS, van Nieuwenhuizen O, Dijkhuizen RM, de Graaf RA, Braun KP. Epilepsia. 2011;52(4):841-5.
MRI of monocyte infiltration in an animal model of neuroinflammation using SPIO-labeled monocytes or free USPIO. Oude Engberink RD, Blezer EL, Hoff EI, van der Pol SM, van der Toorn A, Dijkhuizen RM, de Vries HE. J Cereb Blood Flow Metab. 2008;28(4):841-51.
Magnetic resonance imaging in experimental models of brain disorders. Dijkhuizen RM, Nicolay K. J Cereb Blood Flow Metab. 2003;23(12):1383-402.