The brain is a highly organized complex network with various connections and interactions between neurons. Brain disorders can lead to significant dysfunction of neural networks and consequent functional disabilities. On the other hand, the brain is also capable of reorganizing (through plasticity), which may contribute to (partial) repair and recovery.
Functional MRI (fMRI) is a powerful tool to assess activity in functional brain networks. Functional MRI studies in patients can provide important insights in how brain activation patterns alter and how this relates to functional impairment and recovery. In parallel, fMRI in animal models allow experiments under more controllable and reproducible conditions, which can contribute to elucidating patterns and mechanisms of network remodeling in brain diseases. Several fMRI paradigms are available to assess neural activity and functional connectivity during task execution, (non-invasive) brain stimulation, pharmacological stimulation, or resting state conditions. In addition, functional neuroimaging in small rodents can be conducted with novel optical imaging methods, based on in vivo detection of intrinsic optical signals or fluorescent markers, which can be used to measure hemodynamic and/or electrophysiological activity in cortical tissue.
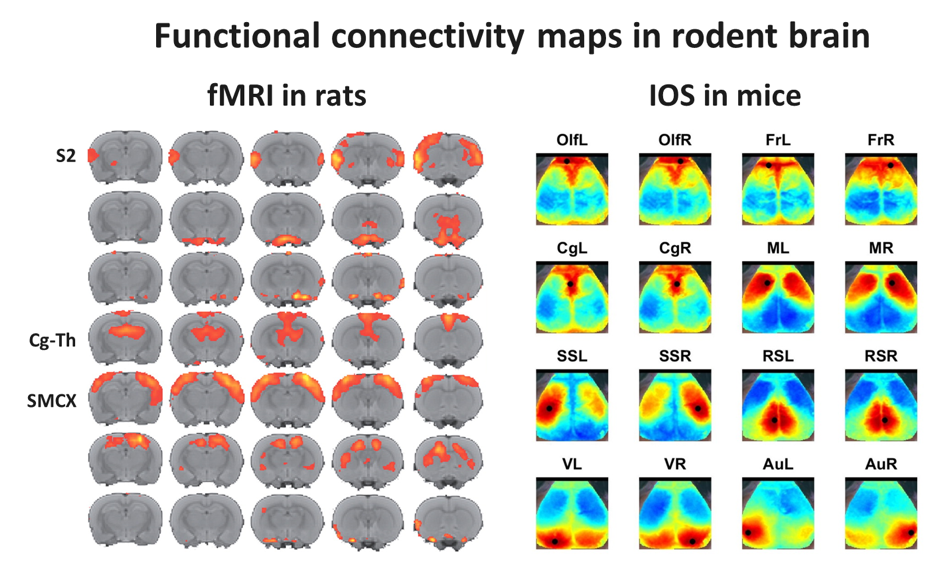 Left: Multislice maps of functional connectivity clusters as calculated by probabilistic independent component analysis of resting-state fMRI data from 20 rats based on 7 components (color-coding reflects Z-scores). Several functional networks can be identified, including the bilateral secondary somatosensory cortices (S2), a bilateral cingulate-thalamic network (Cg-Th), possibly reflecting the default mode network, and the bilateral sensorimotor cortical network (SMCX).
Left: Multislice maps of functional connectivity clusters as calculated by probabilistic independent component analysis of resting-state fMRI data from 20 rats based on 7 components (color-coding reflects Z-scores). Several functional networks can be identified, including the bilateral secondary somatosensory cortices (S2), a bilateral cingulate-thalamic network (Cg-Th), possibly reflecting the default mode network, and the bilateral sensorimotor cortical network (SMCX).
Right: Resting-state functional connectivity maps from mouse brain cortex, acquired with optical imaging of intrinsic signal (OIS). Maps represent functional fields with significant temporal signal correlation with a central cortical region (black circle) (Olf=Olfactory, Fr=Prefrontal, Cg=Cingulate, M=Motor, SS=Somatosensory, RS=Retrosplenial, V=Visual and Au=Auditory; L=left, R=right). Courtesy of Maurits van Meer, Kajo van der Marel, Wim Otte and Geralda van Tilborg.
Alterations in the organization of functional brain networks are dependent on the configuration, stability, and flexibility (plasticity) of underlying macro- and microstructural architecture. This may be assessed with structural MRI methods such as manganese-enhanced MRI, a method for in vivo neuroanatomical tract tracing, and diffusion tensor imaging (DTI). DTI informs on the three-dimensional displacement of tissue water, which can be exploited to assess tissue integrity. The DTI-derived diffusion anisotropy and principal diffusion direction can be used to model and map (tractography) the architecture of neuronal fibers. An alternative method, diffusion kurtosis imaging, allows quantification of the degree to which tissue water diffusion is non-Gaussian, which enables further characterization of the complexity of tissue microstructure.
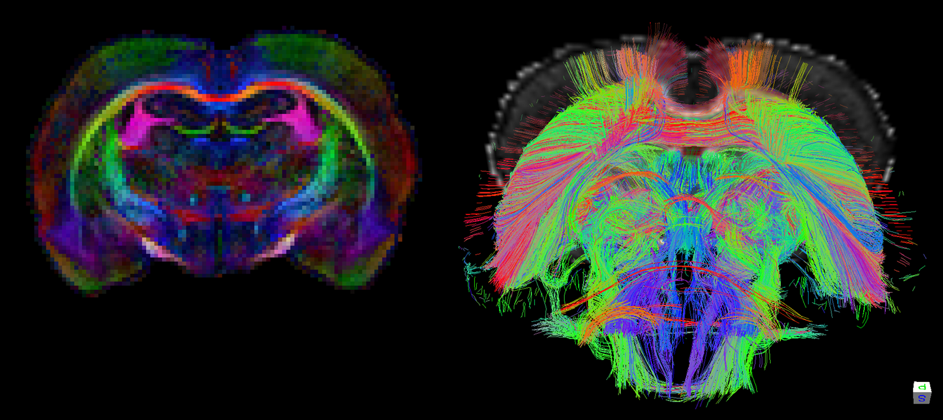 Color-coded maps of fractional anisotropy (FA) (left) and fiber reconstructions (right) in rat brain based on ultrahigh-resolution diffusion tensor imaging (DTI) of ex vivo rat brain at 9.4 T. Courtesy of Wim Otte and Annette van der Toorn.
Color-coded maps of fractional anisotropy (FA) (left) and fiber reconstructions (right) in rat brain based on ultrahigh-resolution diffusion tensor imaging (DTI) of ex vivo rat brain at 9.4 T. Courtesy of Wim Otte and Annette van der Toorn.
With state-of-the-art tools and strategies for analysis of neural networks, based on structural and functional imaging data from human patients and animal models, we aim to elucidate the complex process of brain (re)organization in cerebrovascular diseases, traumatic brain injury, epilepsy and neurodevelopmental disorders. This is done in close collaboration with colleagues from the departments Neurology and Translational Neuroscience at the University Medical Center Utrecht. By combining MRI with behavioral testing, electrophysiological measurements, in vivo optical microscopy, and histology, we try to elucidate the unknown, but hypothetically fundamental, relationships between (changes in) motor/sensory/cognitive function, large-scale neural network (re)organization and micro-scale neuroanatomical connectivity.
Relevant publications
A systematic review on the quantitative relationship between structural and functional network connectivity strength in mammalian brains. Straathof M, Sinke MR, Dijkhuizen RM, Otte WM. J Cereb Blood Flow Metab. 2019 Feb;39(2):189-209.
Diffusion MRI-based cortical connectome reconstruction: dependency on tractography procedures and neuroanatomical characteristics. Sinke MRT, Otte WM, Christiaens D, Schmitt O, Leemans A, van der Toorn A, Sarabdjitsingh RA, Joëls M, Dijkhuizen RM. Brain Struct Funct. 2018;223(5):2269-2285.
A novel approach to map induced activation of neuronal networks using chemogenetics and functional neuroimaging in rats: A proof-of-concept study on the mesocorticolimbic system. Roelofs TJM, Verharen JPH, van Tilborg GAF, Boekhoudt L, van der Toorn A, de Jong JW, Luijendijk MCM, Otte WM, Adan RAH, Dijkhuizen RM. Neuroimage. 2017;156:109-118.
Impact of hemodynamic effects on diffusion-weighted fMRI signals. Rudrapatna US, van der Toorn A, van Meer MP, Dijkhuizen RM. Neuroimage. 2012;61(1):106-14.
Pharmacological magnetic resonance imaging of muscarinic acetylcholine receptor activation in rat brain. Hoff EI, van Oostenbrugge RJ, Otte WM, van der Marel K, Steinbusch HW, Dijkhuizen RM. Neuropharmacology. 2010;58(8):1252-7.